PRODUCT LIABILITY LAWSUITS
We Can Help You Get Justice for Drug and Medical Device Injuries
The Problems with Drug and Medical Devices Makers
“Do no harm.” That’s the oath doctors promise to their patients. It’s sacred and the basis of how all of us expect to be treated. But that promise does not always apply to the companies that make and sell medical devices and drugs. Often the oath of “do no harm” is overshadowed by corporate profit margins and greed. While doctors do their best to heal our bodies, sometimes the drugs they prescribe or the devices they use in surgery are what do the most harm.
When you purchase a product, you expect it to be safe to use or consume. If you are injured by a defective product, the company who harmed you should be held accountable.
If you or someone close to you is injured by a drug, medical device, or other product, we may be able to help.
Current Lawsuits
The attorneys at mctlaw represent people in many types of product liability lawsuits, including many high-profile cases such as the ones listed below. However, we litigate many types of product injuries.
We encourage you to reach out if you’ve been injured by any type of device or drug so our attorneys can review your case.

Kratom Lawsuits
The FDA calls Kratom a dangerous substance that’s injured and killed hundreds of people. Kratom acts like an opioid and can lead to overdoses and serious side effects. The attorneys at mctlaw are filing lawsuits across the US against manufacturers and distributors of this deadly powder.
Metal on Metal Hip Replacements
Patients in jury trials across the country have won multimillion-dollar judgments for injuries caused by defective metal on metal hip replacements. Mctlaw is one of the few law firms leading this litigation.
Vaccine Injury Compensation
Our attorneys have won hundreds of millions of dollars for our vaccine injury clients.
Find out about the National Vaccine Injury Compensation Program and how we can help you file a claim.
Ozempic, Wegovy & Similar Drugs
GLP-1 (Glucagon-Like Peptide-1) drugs are a class of medications used to treat Type 2 diabetes and, more recently, for weight loss. These drugs help regulate blood sugar levels and promote weight loss by mimicking the effects of the GLP-1 hormone, which stimulates insulin production and slows down digestion.
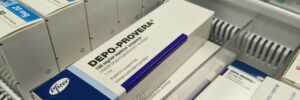
Depo-Provera and Brain Tumor Risk
Recent research has shown a possible connection between the use of Depo-Provera, a popular birth control shot, and an increased risk of brain tumors, specifically meningiomas.

Food Poisoning Product Liability Lawsuits
Millions of people get sick with food poisoning each year. If you were severely injured by contaminated food, find out if mctlaw can help.
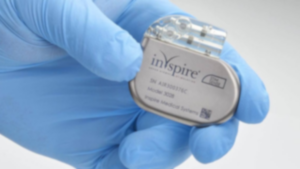
Inspire Sleep Apnea Device Recall
Inspire Medical has recalled its nerve-stimulating sleep apnea implant because of a serious electrical issue. Patients have experienced device failures, forcing them to undergo a revision surgery to remove or replace the implant.

Other Defective Medical Devices and Drugs
Talk to our legal team if you’ve been injured by a medical device or drug. We evaluate various types of medical product liability cases.
What are Medical Devices?
A Medical Device is anything used by a health care practitioner to diagnose, mitigate, treat, or prevent disease, injury or other medical conditions. The FDA classifies medical devices into three categories based on their risks:
CLASS I: The most common type of medical device. These have low to moderate risk, minimal contact with patient, and a low impact on overall health. They are subject to the least amount of government oversight. Examples are elastic bandages, tongue depressors, manual stethoscopes, bedpans, and hospital beds.
CLASS II: These devices are more likely to be in contact with a patient and have moderate to high levels of risk. The government and FDA has more rules over how these items are approved before selling them for use in patients. This is called the 510K process. Examples are powered wheelchairs, catheters, blood pressure cuffs, surgical gloves, and contact lenses
CLASS III: These are products that the FDA defines as “ sustaining or supporting life, are implanted or present a potential unreasonable risk of illness or injury.” Class III devices must go through the toughest FDA scrutiny before going on the market. Examples include defibrillators, artificial hips, shoulders, knees, hernia mesh, breast implants, cochlear implants, and birth control devices.

Talk to our legal team
Your medical product or drug injury might be the first of it’s kind. Or you may not know about an FDA recall or safety alert. A quick conversation with our legal team may help you figure out what to do next.
Product Liability Lawsuits Happen for One of Three Reasons
1. Defective Manufacture
Problems can happen in the factory when the device is being made. These mistakes are called manufacturing defects. There are a lot of ways things can go wrong here. There can be problems with the sterilization process, issues with the quality of the materials used, damage during shipping, or errors at any point between making the device and when you receive it.
2. Defective Design
Design defects happen because of a flaw in the planning and development of the product itself. This type of defect makes it unreasonably dangerous to the consumer.
3. Defective Marketing – Failure to Warn:
Everyone has the right to know the risks and benefits of the products they use. If the manufacturer does not give proper warnings about the dangers of a device, or if it does not explain how to safely use a product, then the company could be liable, and held responsible for an injury. This is called “a failure to warn.”
Manufacturers must also warn health care professionals, like your doctor or surgeon, about risks and how to safely use their medical products. That’s important because doctors often make recommendations to a patient about what types of device or drugs to choose.
Who is Responsible for Your Injury?
These are really complicated cases and might involve more than one defendant in a lawsuit. Possible defendants to hold responsible are:
- The designer of the product
- The manufacturer of the product
- The business or people who marketed the product

About mctlaw
The attorneys at mctlaw represent clients against multinational corporations and governments every day, winning substantial settlements and judgments for our clients.
In the past few years alone, we’ve recovered hundreds of millions of dollars for our clients. We are trial attorneys with a reputation for standing firm when other law firms back down.
We don’t blink, and our clients benefit.
Content Reviewed by Michael Cowgill – Product Liability Lawyer

Michael Cowgill is an experienced attorney in the product liability division at mctlaw. Michael focuses his practice on defective medical devices such as recalled metal-on-metal hips and wrongful death lawsuits involving Kratom. Mr. Cowgill graduated Magna Cum Laude from Lewis & Clark Law School in Portland, OR. He volunteers as a high school mentor with a program for underprivileged youths interested in pursuing a future legal career.
This page was last updated:


Contact Us Now

As an experienced leader in these types of lawsuits, we were confident the firm would have the expertise. However, what surprised us most was the high level of excellent customer service from the firm’s staff!
Pat R.
I can’t recommend this firm enough. They have an outstanding team that truly care for their clients…I have been awarded a fair six figure settlement.
Nate M.
When I say “they went to bat” for me…this Law Firm literally did just that. They persevered to bring the hard-nosed Manufacturer to settle and provide me some recompense for everything I had to endure which led to this suit.
Me’Chelle